The COVID-19 pandemic disrupted the traditional clinical trial model, requiring a virtual approach. Now, in the post-pandemic world, we see continued and expanded adoption of a highly customized and flexible decentralized trial model. Leveraging virtual trial tools and strategies supports our patient-centric approach by reducing the burden on patients while continuing to deliver efficient and superior quality clinical trials.”
- Shaheen Limbada, Executive Vice President, Innovation and Strategy, Veristat
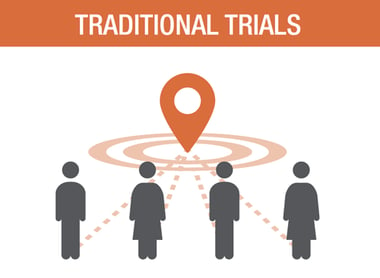
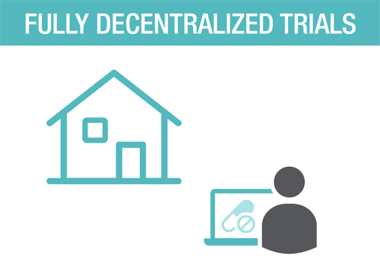
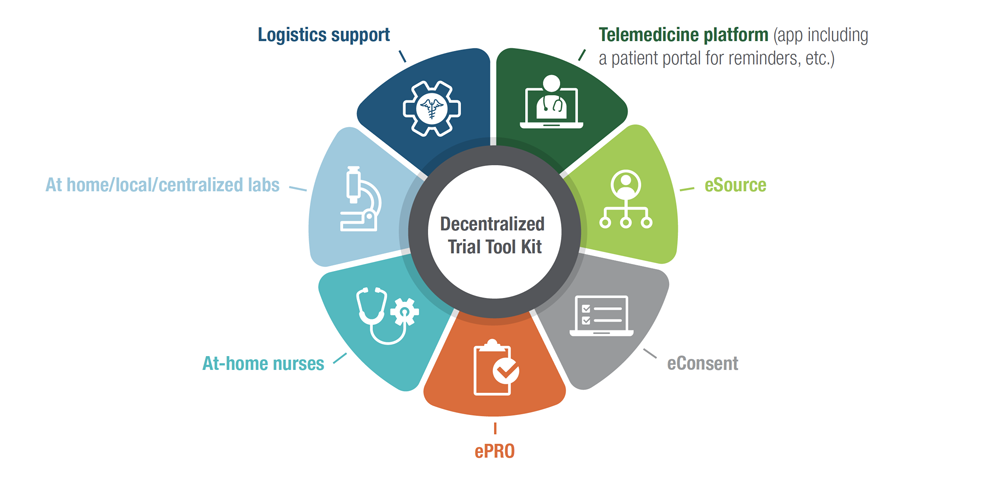
THE DECENTRALIZED TRIALS ECOSYSTEM TOOL KIT, INCLUDING
SPECIALIZED TECHNOLOGY:
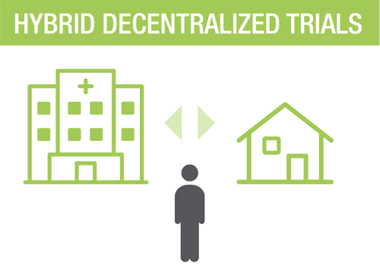
THE FULL EXPERIENCE - HYBRID DECENTRALIZED TRIAL MODEL LISTEN >
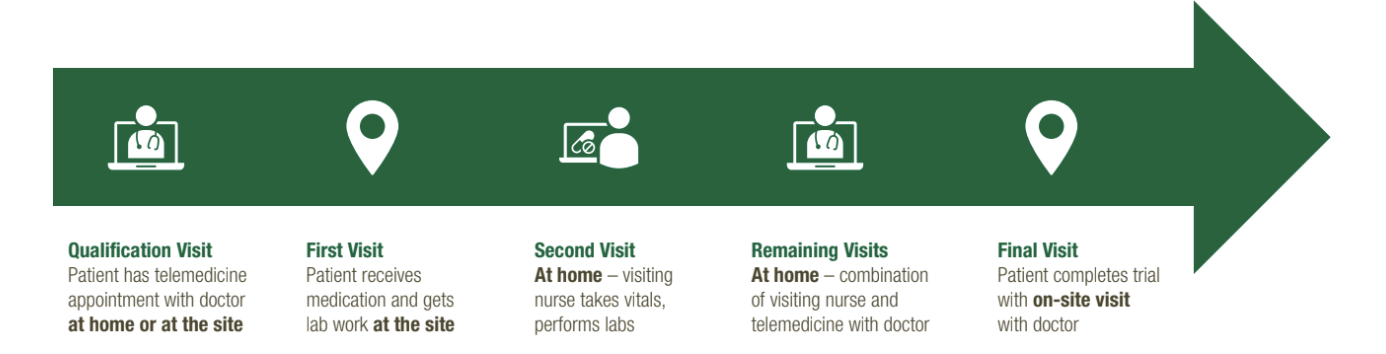
The Patient's Journey Through the Virtual Clinical Trial Experience
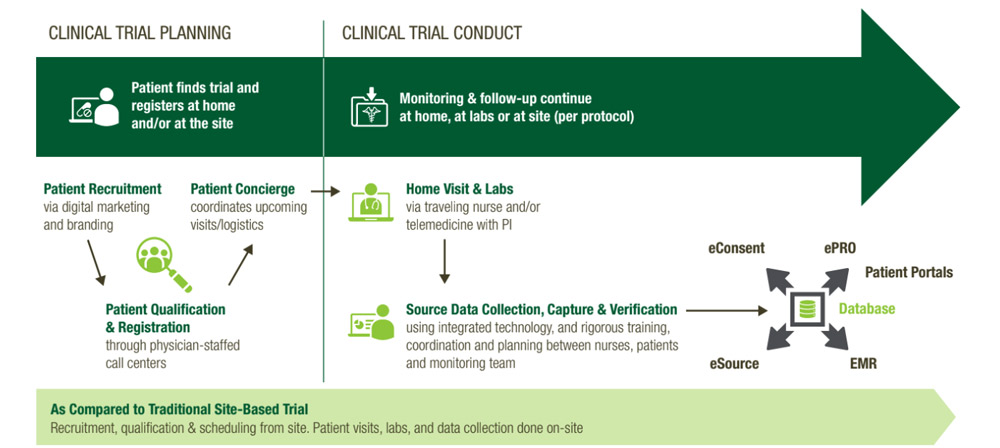
A Hybrid Decentralized Approach Gives You Flexibility
The hybrid solution is completely customizable to the needs of the patients, sites, and protocol. This is just one example – there can be any combination of visits at the site, at-home, or both throughout the duration of the trial.
DECENTRALIZED TRIAL BENEFITS LISTEN >
![]()
Improves patient-centricity of trial by reducing travel/time burden on patients
![]()
Broadens access to patient populations to be more diverse and inclusive – patient access is less restricted by geography
![]()
Improves flexibility – trials can be customized to be completely site-less,
all onsite or a hybrid
![]()
Potentially saves time and cost across development from reduced cycle times, improved patient screening and enrollment, and fewer protocol amendments over the life of a trial (as revealed by a recent Tufts CSDD study).
Overall, decentralized trials improve patient recruitment and retention, which, in
turn increases trial efficiency to save time and cost.
Meet Veristat – Implement Your Decentralized Trials Right, the First Time
We understand how challenging it is to adopt a new way of conducting clinical trials. Pivoting to decentralized clinical trials is no exception. We have the agility and scientific-minded experts to help you navigate this novel approach to clinical development.