Services
Adaptive Design Clinical Trials
Accelerate Clinical Development Success with Proven Expertise for Adaptive Clinical Trial Design, Simulation, and Execution
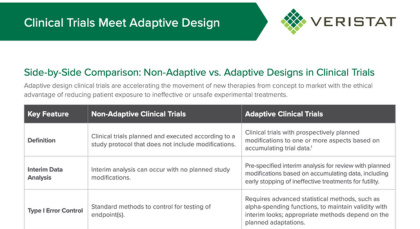
Adaptive design clinical trials are encouraged by Regulatory Agencies, including FDA, to expedite and improve efficiencies for identifying the clinical benefit of new therapies. Adaptive clinical trial designs are more complex than traditional clinical trial designs and therefore, require careful consideration, rigorous planning, and trial simulations before clinical study start-up. But, adaptive designs offer many potential benefits, such as increasing the trial's probability of success, reducing the overall time and cost of the trial, and delivering the right drug to the right patients.
Considering an adaptive design for your next clinical trial?
Adaptive Design Clinical Trials - Defined
Cross-functional expertise is required for the strategic planning, design, optimization, and implementation of your adaptive design. To embark on an adaptive design trial requires the ability to plan and communicate the design to the regulatory agencies and then to communicate and execute the study with the investigational sites and overall project team. Our experts will:
-
Utilize trial simulations to recommend various adaptive design options according to the disease area and company portfolio
-
Develop the adaptive design protocol and outline the strategy for working with regulatory authorities
-
Execute the adaptive design including trial monitoring, interim analysis, and suggesting necessary adaptations
-
Provide analysis and interpretation of the adaptive trial data
-
Manage the independent data monitoring committee (iDMC) activities
"An Adaptive Design is defined as a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial. "
FDA Final 2019 Guidance
Adaptive Designs for Clinical Trials of Drugs and Biologics
-
Let's Talk.
Contact Veristat Today!We know there are always unknown challenges when bringing a novel therapeutics to market, so we’ve assembled an extraordinary team of scientific minded experts who have mastered the complexities of therapeutic development enabling sponsors to succeed in extending and saving lives.
Benefits of Adaptive Design Clinical Trials
-
Reduces cost, time, and required resources - reducing time to market
-
Increases the chance study success via midcourse corrections
-
Mitigates risks by improving patient safety
-
Facilitates better decision-making
Considerations
-
Requires more intensive planning
-
Commands more frequent interaction with regulatory agencies
-
Improper execution can introduce bias
-
Confounding can result from adaptations
-
Certain adaptive designs may require larger sample sizes
-
Statistical significance (alpha) penalties apply

Planning, Simulating, and Implementing Both Simple and Complex Adaptive Design Clinical Trials
Simple Adaptive Design Types
› Group-Sequential Design (GSD)
› Sample Size Re-Estimation (SSR)
› Adaptive Dose-Escalation Design (ADED)

Complex Adaptive Design Types
› Adaptive Dose-Finding
› Adaptive Randomization Design
› Dropping Treatment Arm
(or Pick-the-Winner Design)
› Seamless Phase I/II or II/III Design
› Population Enrichment Design
(aka adaptive enrichment, biomarker-driven design)
Planning Adaptive Design Clinical Trials
Expertise from start to finish
PLAN
Establish adaptive trial goals and outline the strategy for working with regulatory authorities
DESIGN
Develop the adaptive design methodology and design the protocol accordingly
SIMULATE
Utilize trial simulations to recommend various adaptive design options according to the disease area and company portfolio
IMPLEMENT
Execute the adaptive design trial - inclusive of trial monitoring, interim analysis, and making necessary adaptations. Provide final analysis and interpretation of the trial data.
When to Consider Adaptive Designs for Clinical Trials
While adaptive designs can be advantageous in certain circumstances, they are not suited for every clinical trial. Adaptive designs are highly encouraged for clinical trials in the following applications:
Oncology Trials
Particularly in early-phase dose-finding trial designs that use intermediate or accelerated approval endpoints for decision-making.

Rare Disease Trials
Limited patient populations can use supplemental data from earlier run trials, disease progression analytical models, or previous adult trials.

Trials with Cardio Risk
Used for safety monitoring to leverage control patients from other sources.