Solutions
Regulatory Submission and Post-Marketing Safety Surveillance
Navigating the Regulatory Review Process to Ensure Safety and Efficacy.
Determining that your drug/biologic/therapeutic is safe and effective in its proposed use(s) is a key trigger to begin planning your marketing application (e.g. NDA, BLA, NDS, MAA, etc). The preparation of your marketing application is a complex process that comes with special constraints, unique partnership dynamics, and a web of challenges due to the timing of data analysis, medical writing, project management, and interactions with the regulatory agencies.
At Veristat, we understand that the stakes are high when you are working towards the approval of your marketing application. To give you an advantage we have assembled a team of scientific-minded experts that is adept at navigating the process of preparing, publishing, and defending your marketing application as well as preparing for post-market safety surveillance.
Preparing for a Successful Approval Process and Safety Planning
The first hurdle to overcome is to build the marketing application timeline. We always advise that you start with the submission date and work the timeline backwards. Ensure that you’ve included enough time for publication processes, changes based on agency meetings, and any downstream effects of potential revision requests. And don’t forget post-marketing updates, annual reports, and safety surveillance requirements.
In an ideal world, the timeline would be linear, as illustrated below, but the reality is that many of these items happen in parallel.
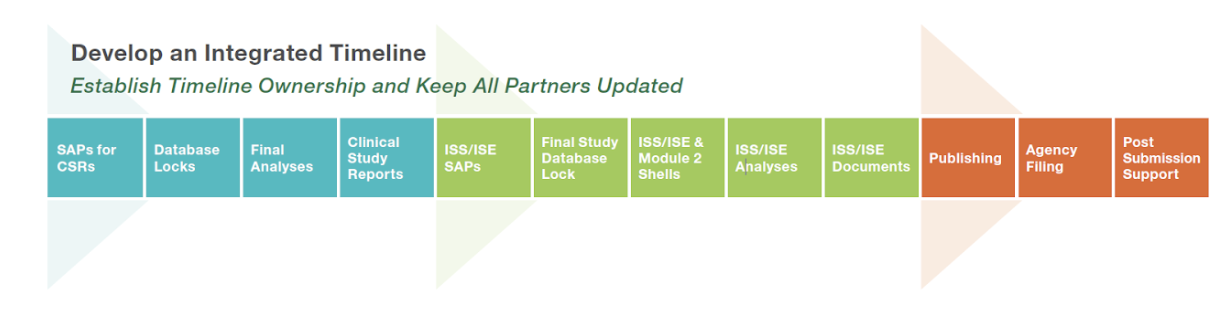
Our regulatory, statistical, medical/safety and communications experts provide real-world experience and knowledge of all the challenges that arise when preparing your marketing application for approval.
Let's Talk.
Contact Veristat Today!
We know there are always unknown challenges when bringing a novel therapeutics to market, so we’ve assembled an extraordinary team of scientific minded experts who have mastered the complexities of therapeutic development enabling sponsors to succeed in extending and saving lives.
We can help:
The Marketing Application Planning Process
![]()
Regulatory & Medical Writing
-
Schedule and prepare for the Pre-NDA meeting
-
Write, edit, and review eCTD modules, CSRs, and Drug label
-
Publish to the NDA to FDA (or appropriate regulatory agency)
-
Respond to agency questions
-
Continued support for safety updates/annual reports
![]()
Data and Statistical Analysis
Standardize the clinical trial data into:
-
SDTM Compliant Datasets
-
ADaM Compliant datasets
![]()
Safety/Pharmacovigilance
-
Develop and negotiate Risk Mitigation plans
-
Post-Market Pharmacovigilance
-
Prepare for agency audits and inspections
-
Set up the PV Systems, processes and training
Avoid Unknowns with the Right Expertise
We understand that the stakes and pressures are high when you are preparing, submitting, and waiting for approval of your marketing application. The submission process is an overwhelming coordination effort. We have the experience, agility, and scientific-minded experts to help you navigate this process successfully and to monitor your product’s safety for its duration on the market.
When you work with Veristat, you get:
-
An agile interdisciplinary team that is adept at working together to prepare and publish your marketing application
-
Achieve your submission timeline- no matter the disruptions
-
The right expertise at the right time (access to expertise when and where you need it)
-
A solid agency relationship with Regulatory Agencies to present and defend your marketing application
-
Peace of mind – this is what Veristat does best!
More Marketing Application and Pharmacovigilance Support |
|||
|---|---|---|---|
|
Marketing Applications
|
Data Standardization
|
Medical Writing
|
Regulatory Agency Meeting Support
|
|
Regulatory Publishing
|
Project Management
|
Pharmacovigilance and Post-Marketing Safety Surveillance
|
|





