Solutions
DCT = Patient Enablement
Optimized and integrated Decentralized Clinical Trials put the patient experience first
Decentralized clinical trials (DCTs) have made it to the mainstream in our ever-evolving world. Yet, for the development of novel therapies that target complex and rare diseases designed for narrowly defined patient populations, DCTs are largely considered difficult to conduct given the many logistical and data challenges.
DCT is not technology
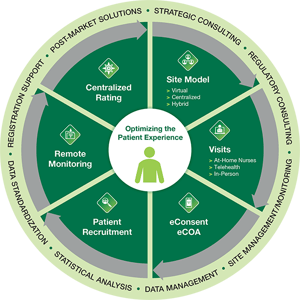
Our cutting-edge solutions ease the burden on patients and sites by integrating remote monitoring, patient reported outcomes, hybrid or virtual trial models, e-consent and at-home nursing, the ideal mix for your study. At Veristat, our clinical and regulatory experts have paved the way in evolving clinical trial conduct for advanced therapies by taking the best elements from traditional, hybrid, and fully decentralized trial methodologies and incorporating them into a unified approach across the drug development journey.
Take patient centricity to the next level with DCT solutions designed specifically for complex studies that reduce the burden on sites and decrease study timelines.
Solving Challenges with Customized DCT Solutions
Veristat has a broad range of expertise conducting trials for cell, gene, rare, pediatric and oncology studies that are small, unique and complex. Our integrated decentralized clinical trial approach is both flexible and customizable to the needs of each patient and trial design. For example, traditional on-site activities such as dosing, lab work, ePRO collection, clinical assessments, medical history, safety visits and follow-up can be conducted remotely at home or through a hybrid model that combines both on-site and at-home visits. In some cases, the investigational product (IP) may be shipped directly to the patient or brought to the patient by a traveling nurse. In other situations, nurses conduct at-home visits to dose patients. The investigator/doctor can conduct some visits with the patient through Telemedicine as well. The customizations are infinite.
Let's Talk.
Contact Veristat Today!
We know there are always unknown challenges when bringing a novel therapeutics to market, so we’ve assembled an extraordinary team of scientific minded experts who have mastered the complexities of therapeutic development enabling sponsors to succeed in extending and saving lives.
Integrating DCT Solutions Into How We Run Clinical Trials
Address the challenges of running studies with limited patients available:
-
Improves patient recruitment, inclusion and retention by reducing the patient burden of travel and time going to the physical site.
-
Finds the right patients by removing geographic barriers to patient eligibility
-
Improves flexibility and overall efficiency with customized trials that are completely virtual/decentralized, all onsite, or a hybrid of both
-
Offers a fully integrated model customized to the comfort levels of the patients, sites, sponsors, etc.
-
Removes silos between sites, sponsor and CRO. Integrated into the conduct of our clinical trial execution
-
Increases accessibility to all eligible patients with approaches that address individualized needs for greater diversity
How to Make a Decentralized Trial Toolbox Work?
A decentralized clinical trial is more complicated than sending a traveling nurse to visit a patient at their home. Our comprehensive and full-service approach to virtual clinical trial conduct integrates all the processes and technologies necessary for success. We coordinate all communication channels between patients, sites, nurses, labs, and trial monitors.
Virtual clinical trial success requires an integrated approach:
- Developing a digital marketing and branding strategies for patient recruitment and education
- Mobilizing physician-staffed call center to qualify and register patients
- Managing communication channels between the site, at-home nurse, patient, and other integrated vendors/technologies
- Performing study protocol requirements by traveling nurses. A nurse conducts the at-home patient visits as outlined by the study protocol
- Managing logistics differently - Proven logistics management such as sending supplies to the patient instead of site, facilitating lab work, etc.
- Collecting source data at home
- Connecting patients and treating physicians through Telemedicine (video meetings)
- Utilizing ePRO, eSource, and eConsent capabilities that integrate with EDC
- Monitoring traditional sites and virtual sites remotely

Is a Decentralized Clinical Trial Approach Right for You?
We understand how challenging it is to adopt a new way of conducting clinical trials. Pivoting to decentralized clinical trials is no exception. We have the agility and scientific-minded experts to help you navigate this novel approach to clinical development.
With numerous decentralized trials running right now, we have designed a customized ecosystem to satisfy both the study and patients' needs.