Solutions
Full-Service Clinical Trial Delivery
Accelerate your novel medical therapies from Phase I-III clinical development to market with confidence.
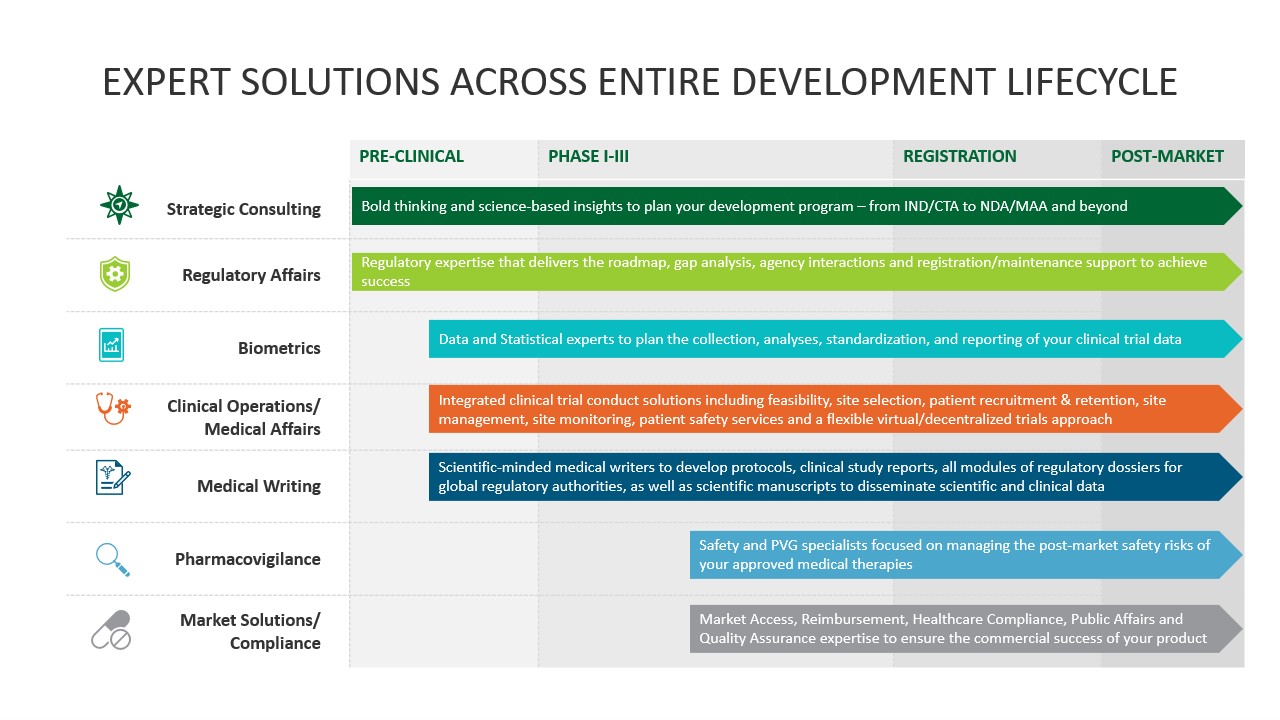
Getting a novel therapy through the clinical development process to approval and commercialization is complicated and full of challenges. Veristat has assembled an extraordinary team of scientific-minded experts who strategically design and execute clinical trials and prepare your clinical data for regulatory review. Our integrated regulatory, clinical, biometrics, safety, and medical writing experts support small to mid-sized biopharma companies running their development programs throughout North America, Europe, and many other regions worldwide.
Helping you make and implement the right decisions at the right time is our strength. We help you overcome your challenges and advance your compound to the next step in the clinical development process.
Want to accelerate your clinical trial or development program?
In the last 5 years, Veristat teams kicked off 100+ full-service clinical trials, of which nearly 50% were for biologics and >30% were to treat patients with rare diseases.
Let's Talk.
Contact Veristat Today!
We know there are always unknown challenges when bringing a novel therapeutics to market, so we’ve assembled an extraordinary team of scientific minded experts who have mastered the complexities of therapeutic development enabling sponsors to succeed in extending and saving lives.
Accelerating Success in the Clinical Development Journey
Avoid Unknowns from the Start
We are here to establish patient safety throughout clinical trial planning and conduct, navigating the regulatory approval process, and post-marketing surveillance. When you start thinking about your first-in-human trials or have solved all the complex challenges and do not know how to move forward, it's time to contact us. Veristat has the right resources to help you navigate:
Adopting Decentralized Trial Strategies
Accelerating patient recruitment and easing the patient participation burden is now common through the deployment of virtual trial tools.
Our clinical trial teams seamlessly incorporate technologies and solutions based on the custom needs of each trial.
The majority of all clinical trials today are hybrid, meaning they include virtual or decentralized components.
Scientific Minds Solving Your Critical Problems
Our highly qualified scientific-minded experts aren’t simply executing the clinical trial plan. They are strategic thinkers who evaluate challenges, collect data, plan with multiple options, weigh pros and cons, balance short—and long-term goals, and identify the risks involved with every option.
The proof is in our full-service delivery of more than 100 clinical trials in the past 5 years:
-
>50% of clinical trials are for complex biologics
-
>30% of clinical trials are for rare/ultra-rare diseases
-
>65% of global operations team members have advanced degrees