Why Veristat
Science-First Approach to Clinical Development
Working Together to Solve Complex Challenges and Deliver Results.
What makes for a positive client experience? At Veristat, we believe it is based on our ability to accomplish more, more effectively and with less worry for you. With flexible and scalable engagement models and unparalleled expertise, we support your success by orchestrating and conducting activities that accelerate access to high-quality therapies globally.
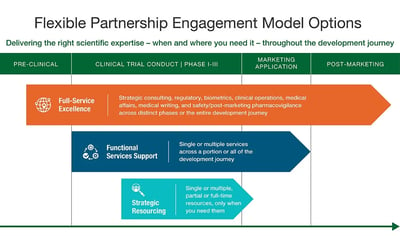
Tailored Engagement Models to Support Success
Full Service Solutions
End-to-end capabilities across the development journey
Overcome the challenges that arise during clinical development, regulatory approval, and post-marketing.
Functional Service Solutions
Targeted support centered on expertise
Count on Veristat’s high-performing functional experts using best-in-class SOPs to increase quality and accelerate time to market.
Strategic Resourcing Solutions
Engagement-based solutions designed to limit risk & accelerate milestones
Manage expert Veristat resources exactly when and where you need.
Let's Talk.
Contact Veristat Today!
We know there are always unknown challenges when bringing a novel therapeutics to market, so we’ve assembled an extraordinary team of scientific minded experts who have mastered the complexities of therapeutic development enabling sponsors to succeed in extending and saving lives.
Veristat has been a trusted source of intellectual leadership and scientific-minded guidance in clinical development to guide your critical decision-making. We provide strategic level thinking to help clients optimize their clinical development approaches, program planning/trial designs and regulatory submission strategies. Learn More
We can project manage every aspect of your trial or just provide supplemental services to round out your team. Our experts collaborate with you by providing strategic consultation throughout your program, clinical operations support, safety management, data management, CDISC implementation, biostatistics, statistical programming, medical writing and regulatory submissions support. Learn More
Our project managers, medical writers, data managers, data standards team, biostatisticians, statistical programmers, and regulatory affairs experts work tirelessly in unison to successfully prepare regulatory strategies and marketing applications. As testament, our teams have prepared over 160 regulatory marketing applications that have led to more than 80 regulatory approvals to date. We executed a flawless regulatory submission process which helped lead to their approval. Learn More
Our breath of experts supports you beyond the approval milestone. Continued post-marketing pharmacovigilance, market access & reimbursement, public affairs and legal/healthcare compliance are critical to keep your therapy on the market and available to the patients who need them. Let us Help
Making A Difference Everyday
Our teams are passionate and committed to guiding you through a successful clinical trial and regulatory submission process. We want to help you bring therapies to market to improve lives. Learn how we help our clients achieve success!


