Overcoming Clinical Trial Challenges for Blood Disorders
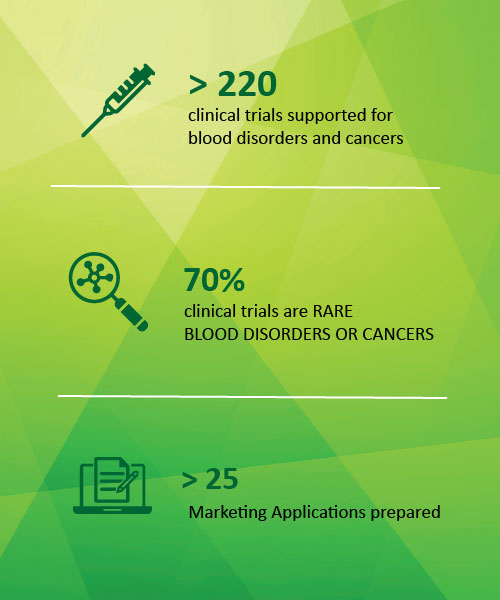
Veristat has guided clients through the conduct of more than 250 projects and over 30 marketing applications for blood diseases. Our experience spans clinical development consulting, full clinical trial oversight, and regulatory submission preparation for rare genetic blood disorders and autoimmune blood diseases, as well as blood cancers.
At Veristat, our experienced teams are poised to plan and implement these efforts quickly providing:
- Development of regulatory strategy, expedited pathways, and regulatory agency interactions
- Clinical program planning, inclusive of statistical planning and analysis
- Agile patient recruitment strategies to support virtual trial and hybrid approaches
- Rapid deployment of clinical trial databases
- Virtual and remote clinical and medical site monitoring
- Data analysis and migration into CDISC formats
- Writing of clinical trial, safety, and regulatory documents
- Preparation and defense of Marketing Applications – NDAs, BLAs, NDSs, MAAs, jNDAs, etc.