Veristat Resource Library
Considerations for Enhancing Clinical Trial Efficiency Through Database Design
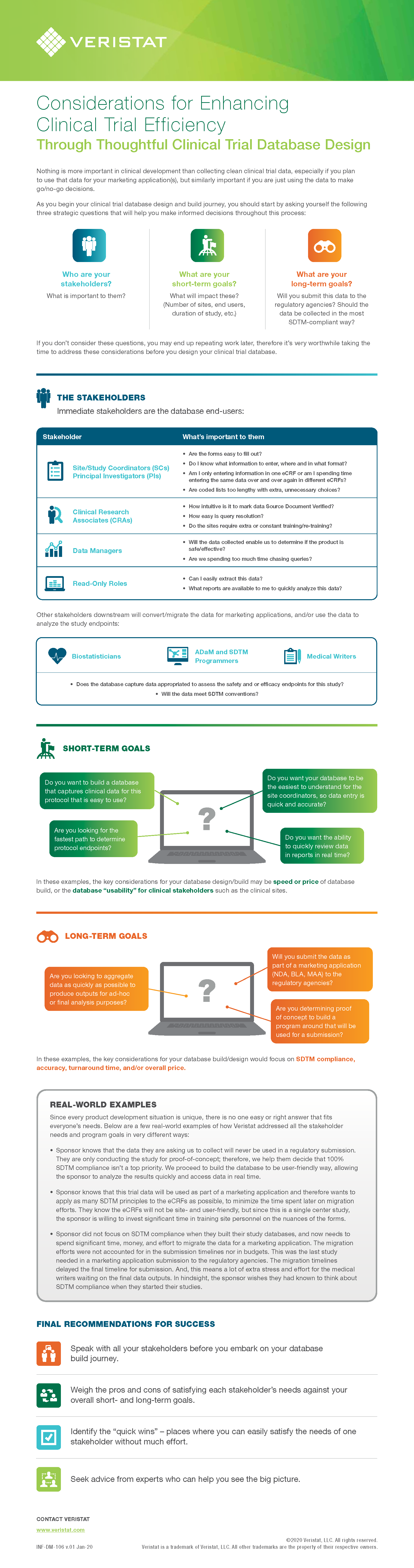
Designing a clinical trial database is a balancing act. Any clinical program has multiple stakeholders who have both short-term and long-term goals to achieve. A good database designer must understand the needs and goals of the sponsor and its various functional project team members. The designer must consider the ease of use for the clinical trial sites who will be entering the trial data, compared to the needs of the downstream stakeholders in biostatistics, programming, and medical writing. Additionally, not only do today's priorities need to be considered, but the overall strategy of the clinical program must also be understood, especially as it moves forward past the current stage.
Get the infographic to better understand the key considerations that must be discussed prior to designing your clinical trial database, all of which can impact the efficiency of the clinical program, including:
- Identifying the key stakeholders and their needs
- Understanding how database design will affect data migration and conversion in both the short- and long-term goals of your individual trial(s) and program as a whole
- Real-life examples of balancing sponsor/site needs in database design
- Recommendations for successfully balancing all these needs in clinical trials database design
Please fill out this form to access your resource.

