Home >
library
Library
Veristat Resource Library
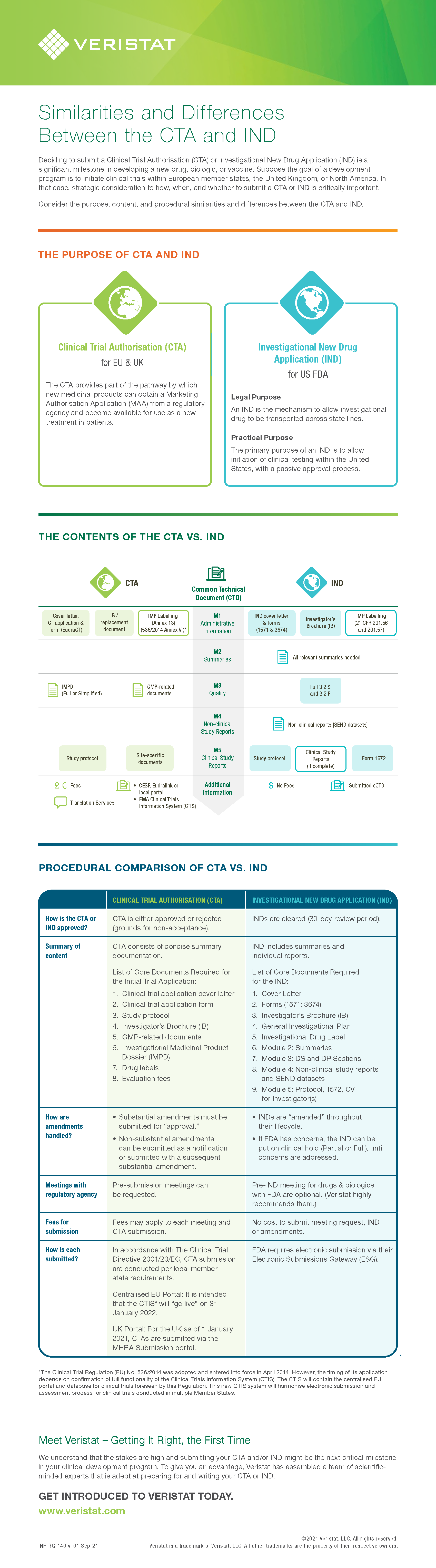
Similarities and Differences Between the CTA and IND
Deciding to submit a Clinical Trial Authorisation (CTA) or Investigational New Drug Application (IND) is a significant milestone in developing a new drug, biologic, or vaccine.
Suppose the goal of a development program is to initiate clinical trials within European member states, the United Kingdom, or North America. In that case, strategic consideration to how, when, and whether to submit a CTA or IND is critically important.
Consider the purpose, content, and procedural similarities and differences between the CTA and IND.
Explore Our Key Related Services
Please fill out this form to access your resource.

