Home >
library
Library
Veristat Resource Library
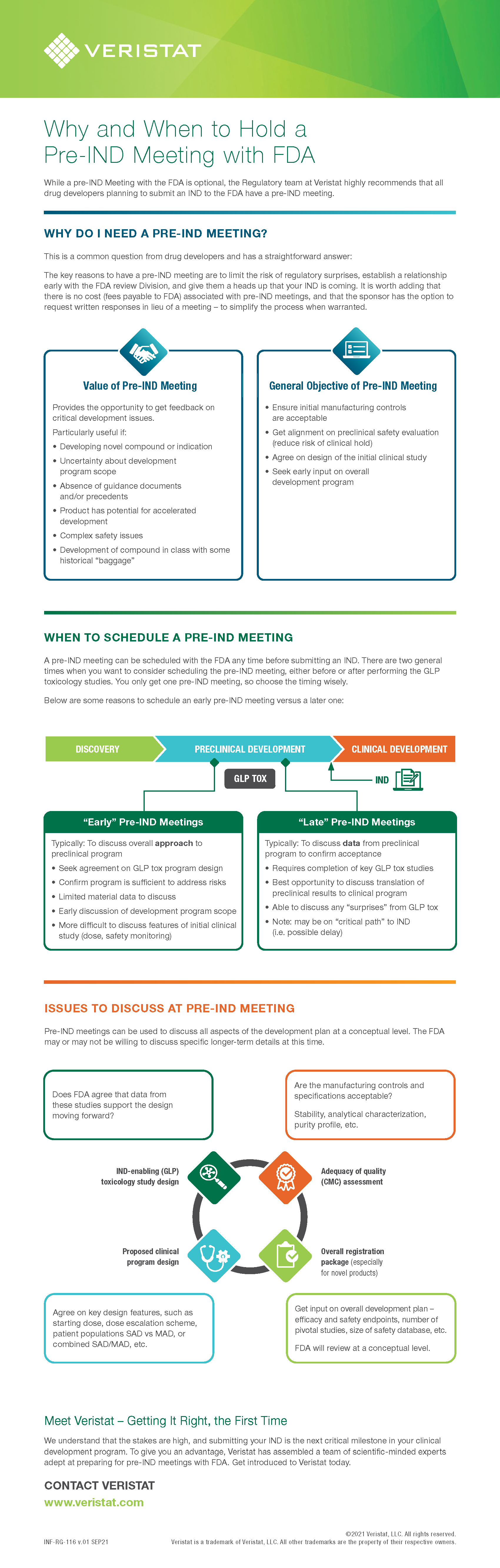
Why and When to Hold a Pre-IND Meeting with FDA
WHY DO I NEED A PRE-IND MEETING?
This is a common question from drug developers and has a straightforward answer:
The key reasons to have a pre-IND meeting are to limit the risk of regulatory surprises, establish a relationship early with the FDA review Division, and give them a heads up that your IND is coming. It is worth adding that there is no cost (fees payable to FDA) associated with pre-IND meetings, and that the sponsor has the option to request written responses in lieu of a meeting – to simplify the process when warranted.
downloadExplore Our Key Related Services
Please fill out this form to access your resource.

