1 min read
SCOPE: Powering the Future of Clinical Research
Meet Veristat at SCOPE: Powering the Future of Clinical Research February 2-5, 2026
🔬 Advancing Clinical Research with...
1 min read
Based on an online seminar presentation made in collaboration with TOPRA and Veristat, this article dives into the future of the European medicines and medical devices industry and the changes in legislation that are being prompted by the evolving pharmaceutical environment. 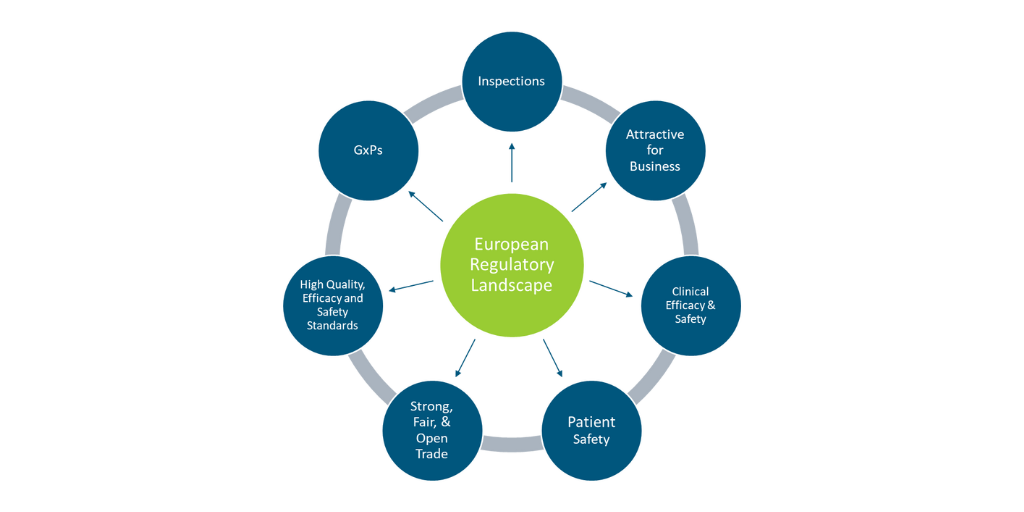
On 25 November 2020, the European Commission (EC) adopted the patient-centric New Pharmaceutical Strategy for Europe and published its roadmap for the revision of the general pharmaceutical legislation in 2021. The European Commission has begun initiating work in several areas, including the pediatric and orphan review.
In this article, you will get a comprehensive overview of the following:
Read the full article to learn more: https://www.regulatoryrapporteur.org/european-medicines-and-medical-devices-future-trends/222.article
1 min read
Nov 20, 2025 Veristat Events
🔬 Advancing Clinical Research with...
1 min read
Oct 22, 2025 Veristat Events
Veristat is excited to attend the ASH Annual Meeting and...