1 min read
SCOPE: Powering the Future of Clinical Research
Meet Veristat at SCOPE: Powering the Future of Clinical Research February 2-5, 2026
🔬 Advancing Clinical Research with...
2 min read
Explore the growing role of Real-World Evidence (RWE) in the regulatory decision-making process for medicinal products in Europe. Learn how RWE is reshaping the development landscape, its potential benefits, and the challenges associated with its implementation.
Real-World Evidence (RWE) is gaining attention in the regulatory industry as a valuable tool for understanding the safety and effectiveness of medicinal products in real-world settings. This blog post explores the regulatory framework for RWE in Europe and how it is used in regulatory procedures and decisions. With insights from Veristat's regulatory affairs experts, discover the potential of RWE to shape the future of medicinal product development. 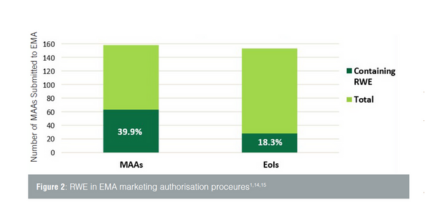
What is Real-World Evidence and its Potential?
Real-World Evidence (RWE) refers to evidence obtained from the analysis of Real-World Data (RWD), which includes electronic health records, patient registries, health applications, and environmental data. RWE offers a comprehensive understanding of a medicinal product's safety and effectiveness by assessing its performance in a broader population and providing long-term data on treatment outcomes. It can also help identify patient subgroups that may benefit from the medicine or are at increased risk of adverse events.
Regulatory Framework in the EU
In Europe, there is increasing support for using RWE in the regulatory framework. The European Medicines Agency's (EMA) Network Strategy to 2025 emphasizes the importance of data analytics and digital transformation, aligning with the European Commission's Pharmaceutical strategy for Europe. The EMA supports using RWE through initiatives like the Data Analysis and Real World Interrogation Network (DARWIN EU®), which aims to provide reliable evidence on medicinal products' use, safety, and efficacy.
Use of RWE in Regulatory Procedures
RWE is considered separately for pre-authorization activities and marketing authorization procedures. The Paediatric Committee (PDCO) and the Committee of Orphan Medicinal Products (COMP) explore using RWE to support paediatric development and rare diseases, respectively. The Committee for Medicinal Products for Human Use (CHMP) has initiated pilot studies on RWE to support scientific advice and evaluate its added value alongside traditional randomized clinical trials (RCTs).
Evaluation of RWE in Marketing Authorization Applications (MAA) and Extension of Indications (EoI) shows that its usage remains relatively low, primarily focused on safety. However, RWE has the potential to provide pre-authorization efficacy evidence and contribute to regulatory benefit/risk assessment. Post-authorization studies are also considered, particularly in conditional marketing authorizations, where RWE can confirm positive benefit/risk assessment.
The Future Role of RWE in Regulatory Decision-Making
While the current usage of RWE in European regulatory procedures is modest, there is a growing potential for RWE to play a more significant role in the decision-making process. Initiatives are underway to establish its value and reliability, and the EMA is actively exploring using RWE during product development. As further research, evaluation, and standardization are conducted, RWE has the potential to enhance patient outcomes and streamline the medicinal product development process.
RWE is poised to shape the future of regulatory decision-making in Europe. With its ability to provide valuable insights into real-world outcomes and patient populations, RWE offers a promising avenue for enhancing the development and use of medicinal products. While challenges exist in establishing its value and reliability, ongoing efforts within the regulatory industry will contribute to the advancement of RWE as a vital tool in regulatory procedures.
Stay tuned for further updates on RWE's impact on medicinal product development in Europe.
Authors:
Paula Meler, Anna Huguet, Elisa Moya, Martina Carol, Regulatory Affairs Professionals at Veristat
Download the full article here:
https://www.calameo.com/read/0061133854538324c72b3?page=45
International Clinical Trials, May 2023, pages 44-46 © Samedan Ltd
1 min read
Nov 20, 2025 Veristat Events
🔬 Advancing Clinical Research with...
1 min read
Oct 22, 2025 Veristat Events
Veristat is excited to attend the ASH Annual Meeting and...