1 min read
SCOPE: Powering the Future of Clinical Research
Meet Veristat at SCOPE: Powering the Future of Clinical Research February 2-5, 2026
🔬 Advancing Clinical Research with...
The In Vitro Diagnostics Regulation (IVDR, EU 2017/746) enters into application in the European Union (EU) on 26 May 2022.
Following the Medical Devices Regulation last year, the entry into application of the IVDR brings significant changes to the framework of in vitro diagnostics (IVDs) in the EU, including:
To address the challenges of the IVDR implementation, including the limited number of NBs, the European Commission proposed a progressive roll-out of the IVDR. Regulation 2022/112, that entered into force on 28 January 2022, amends the transition periods as follows:
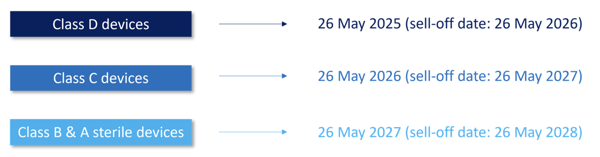
The amended transition periods also impact the implementation of rules regarding the in-house device exemption.
26 May 2022 remains the general application date for the IVDR, in particular for CE-marked IVDs that do not require the involvement of an NB (i.e. Class A non-sterile devices) and IVDs not covered by a certificate or a manufacturer’s declaration of conformity issued prior to this date.
Several initiatives will accompany the implementation of the IVDR:
Our experienced multi-disciplinary team can support you throughout the lifecycle of IVDs, including companion diagnostics, genetic tests, and near-patient testing devices.
SFL, a Veristat company is a leading consultancy with a unique combination of expertise under one roof. Thanks to its experienced multidisciplinary team, SFL’s services cover the entire life cycle of healthcare products. SFL offers personalized and customized strategic advice and operational support of global as well as regional and national projects.
1 min read
Nov 20, 2025 Veristat Events
🔬 Advancing Clinical Research with...
1 min read
Oct 22, 2025 Veristat Events
Veristat is excited to attend the ASH Annual Meeting and...