1 min read
SCOPE: Powering the Future of Clinical Research
Meet Veristat at SCOPE: Powering the Future of Clinical Research February 2-5, 2026
🔬 Advancing Clinical Research with...
4 min read
An adaptive design is a clinical trial design that allows adaptations or modifications to aspects of the trial after its initiation without undermining the validity and integrity of the trial. An adaptive design consists of multiple stages. At each stage, data analyses are conducted and adaptations take place based on updated information to maximize the probability of success of a trial.
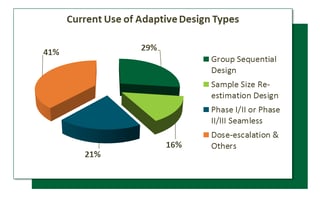
In a recent investigation on the use of adaptive trial designs, it was reported that among all adaptive design types that were reviewed: 29% of studies used Group Sequential Design, 16% of studies used Sample Size Re-Estimation, 21% were Phase-I/II or Phase-II/III seamless designs, and 41% were dose-escalation, dose-selection and a mix of others.
However, in order to fully understand the advantages of adaptive designs, you have to consider the different types of adaptive designs, their applicable situations, and the so-called operating characteristics, such as average and maximum sample-sizes, early stopping probabilities, and the power.
Let’s quickly explore the common types of trial adaptations which include:
A group sequential design (GSD) is the simplest type of adaptive design that allows for premature termination of a trial due to efficacy or futility based on the results of the interim analyses (IA). There are three sub-types of GSDs which are described by their names: early efficacy stopping design, early futility stopping design, and early efficacy/futility stopping design. Statistically, a GSD requires control type-I error, that is the probability of falsely rejecting null hypothesis must be kept below a nominal level. A GSD may save cost and time due to early efficacy claim at the IA when the treatment effect larger than expected or early futility claim when the treatment effect is much smaller than expected.
A GSD has to pre-specify at the design stage the total numbers of analyses to be conducted and the timing of interim analyses. This might not be practical due to e.g., the availabilities of DMC (data monitor committee) members. The Error-Spending Trial design (ESTD) is essentially a GSD, but with the added extra aforementioned flexibilities to allow changes in the total number and timing of the analyses. To control type-I error, an ESTD requires pre-specified error-spending function such that when the timing of the analysis changes, the error-spent at the interim analysis, thus the efficacy stopping boundary will change accordingly. People sometime call ESTD a group sequential design with certain error-spending (e.g., O’Brien-Fleming-like error-spending function). The concept of error-spending can be used in other adaptive designs as well.
A GSD requires the maximum sample size to be pre-specified at the design stage. However, if the treatment effect is under estimated or the interim data show unfortunately low effect, which may cause a trial continuing to the final stage but marginally failed (i.e, p-value just a little over the threshold), we may want to increase the maximum sample size for the final analysis to protect power or conditional power. Such a GSD, featuring sample-size re-estimation at the interim analyses, is called sample size re-estimation (SSR) design.
(also known as drop-the-loser, drop-arm, adaptive dose-finding design, or Phase II/III seamless design) Traditionally, Phase-IIB dose-finding and Phase-III confirmatory study are two independent studies. However, a more efficient design is to combine the two studies into one trial, called Pick-the-Winner design (PWD). Such a design if used properly, can reduce the number of patients required and can shorten the duration of the overall development program for the compound. A PWD is typically a multiple-arm design with two stages: a selection stage and a confirmation stage. For the selection stage, a randomized parallel design with several doses and a placebo group is employed. After the best dose (the winner) is chosen the patients of the selected dose group and placebo group continue to enter the confirmation stage. New patients are recruited and randomized to receive the selected dose (winner) or placebo. The final analysis is performed with the cumulative data of patients from both stages.
Response-adaptive randomization Design (RAR) is a trial design in which the allocation of patients to treatment groups is based on the responses (outcomes) of the previous patients. The main purpose is to provide a better chance of randomizing the patients to a superior treatment group based on the knowledge about the treatment effect at the time of randomization. A RAR is typically not required to control type-I error. Instead, the determination of design parameters are so optimized to, e.g., minimize the number of failures in the trial.
An adaptive dose-escalation design (ADED) is a design in which the dose level used to treat the next-entered patient is dependent on the toxicity of the previous patients, based on some traditional escalation rules. Many early dose-escalation rules are adaptive, but the adaptation algorithm is somewhat ad hoc. Recently more advanced dose-escalation rules have been developed using modeling approaches (frequentist or Bayesian framework) such as the continual reassessment method (CRM) and other accelerated escalation algorithms. These algorithms can reduce the sample-size and overall toxicity in a trial and improve the accuracy and precision of the estimation of the maximum tolerated dose (MTD). In an ADED, we need to define the toxicity rate of MTD for your disease indication because different designs target different toxicity rate. The typical evaluation matrix for ADED are the sample size, the number of patients with toxicities, and the accurate of predicted MTDs. No type-I error control is enforced.
A biomarker-adaptive design is a design that allows for adaptations using information obtained from biomarkers. A biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biologic or pathogenic processes or pharmacologic response to a therapeutic intervention. A biomarker can be a classifier, prognostic, or predictive marker each of which have differing effects on the types of adaptations one might plan in the study. Overall, biomarkers are used at the interim analysis to assist in decision-making, while the final decision will still be based on a primary or gold-standard endpoint, such as survival.
All adaptive trial designers face a choice among different types of adaptive designs, and the choice of design parameters after determination of a certain type of adaptive design. To make an intelligent choice, the design team has to construct an evaluation matrix and ideally a utility function that combine all aspects in the evaluation matrix into a numerical value. The design corresponding to the maximum utility is the optimal design.
This list of adaptive designs is not exhaustive but will provide a quick foundation of understanding of the types of adaptive designs commonly used in clinical trials today. To read more in depth about these types of adaptive designs, when to use them and to explore the opportunities and challenges of each design, read the full article “Adaptive Designs- Recent Advancement in Clinical Trials” by Mark Chang, PhD, Senior Vice President of Strategic Statistical Consulting at Veristat and John Balser, PhD, President and Chief Biostatistician at Veristat.
To read more in depth about these types of adaptive designs, when to use them and to explore the opportunities and challenges of each design, read the full article “Adaptive Designs- Recent Advancement in Clinical Trials” by Mark Chang, PhD, Senior Vice President of Strategic Statistical Consulting at Veristat and John Balser, PhD, President and Chief Biostatistician at Veristat.
1 min read
Nov 20, 2025 Veristat Events
🔬 Advancing Clinical Research with...
1 min read
Oct 22, 2025 Veristat Events
Veristat is excited to attend the ASH Annual Meeting and...